What is Radioactive Decay What is Radioactive Decay Easy Steps
This article discusses about radioactive decay types. We know that atoms are held together by a force called interatomic force or nuclear force.
When an unstable atom wants to attain a stable state, it does so by emitting a large amount of energy through radiation. This extra energy being the reason behind instability of that atom is shredded by the atom itself. This phenomenon is called as radioactivity. We shall read more about radioactivity in this article.
- Alpha Decay
- Beta Decay
- Gamma Decay
- Neutron Emission
- Electron Emission
- Cluster Decay
What is radioactivity?
As discussed in above section, it is the phenomenon in which the unstable atom loses its energy to attain stability.
The energy released is termed as nuclear or atomic energy as it is derived from the nucleus of the atom. We shall study more about radioactivity and its types in further sections of this article.
Radioactive decay types
There are many ways in which the nuclear energy can be emitted. The different types of radioactive decay are listed below-
Alpha decay
Alpha particles are those particles which consists of two protons and two neutrons (Like He atom). When the nucleus emits alpha particles in a reaction then it is called as alpha decay.
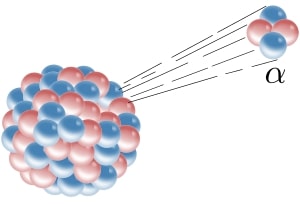
Image Credits: Wikipedia
Beta decay
Just like Alpha decay, in Beta decay, the Beta particles are emitted. Beta particles are those particles which have a pair consisting of positron and neutrino or electron and anti neutrino. When positron and neutrino are emitted, it is termed as beta plus decay and similarly when electron and anti neutrino are emitted it is termed as beta minus decay.
Gamma decay
Gamma decay takes place in two steps. First nucleus emits Alpha or Beta particles and leaves the nucleus in an excited state. To achieve a stable state, nucleus emits gamma ray photons. This is called as gamma decay.
Neutron emission
In some cases, due to excessive Alpha Decay or Beta Decay, the remaining nuclei become neutron rich. These neutrons are shedded away by the process of neutron emission. This results in formation of Isotopes of different particles.
Electron capture
Sometimes, Nucleus may capture an orbiting electron. This leaves the proton alone due to which it gets converted to neutron. During this process, Neutrino and gamma rays are emitted.
Cluster Decay
In Cluster decay a nucleus heavier than Alpha particle is emitted.
Radioactive decay series example
An unstable atom undergoes a series of radioactive decays or transformations to attain a stable state. This series of transformations is termed as radioactive decay series.
A radioactive decay series is also called as radioactive cascade, the atom does not get directly converted to a stable state. Rather it undergoes many transformations to reach a stable state. Examples of radioactive decay series is given below-
- Thorium series– In Thorium series, following elements are present- Actnium, Bismuth Lead, Polonium, Radon, Radium and Thallium. The total energy release from Thorium-232 to Lead-208 is 42.6 MeV.
- Neptunium Series– In Neptunium series, only two isotopes are involved namely Bismuth-209 and Thallium-205. The total energy release from Californium-249 to Thallium-205 is 66.8 MeV.
- Uranium Series– Uranium series contains the following elements- Astatine, Bismuth, Lead, Polonium, Protactinium, Radium and Radon, Thallium and Thorium. The total energy release from Uranium-238 to Lead-206 is 51.7 MeV.
- Actinium series– The Actinium series consists of- Actinium, Astatine, Bismuth, Francium, Lead, Polonium, Protactinium, Radium, Thallium, Thorium and Radon. The total energy released from Uranium-235 and Lead-207 is 46.4 MeV.
Radioactive decay properties
We have discussed in above sections that radioactivity is the phenomenon in which an atom reduces its energy to attain a stable state. The energy released through these atoms is high enough to make an Atom bomb.
The process of radioactive decay is very random, one cannot simply tell which atom is going to disintegrate into which atom. The entire energy release process is spontaneous. The transformation theory does not tell about the particular cause inside the atom which is responsible for the emission of this extra energy.
Radioactive decay uses
Although, humans have a dangerous threat of nuclear radiation. A slight amount of exposure to radiation can cause illness, burns and severe diseases which can lead to death. Excessive amount can cause instant death.
But it can be used in a better way if the energy is harnessed in a proper manner. Let us see some uses of radioactivity-
- Medicine– Cobalt-60 is used extensively to trap cancer cells. This is a major breakthrough in fighting cancer.
- Electricity generation– Uranium-235 is a commonly used fuel in nuclear power plants. Even a small amount of Uranium-235 can be used to generates megawatts of electricity.
- Treatment– Iodine-131 is used in treating hyperthyroidism. Some radioactive isotopes are used in diagnostic purposes as well as for research.
- Measurement of thickness-The strength of penetrations if these radioactive elements can be used precise measurement of thicknesses of plastics and metals in industries.
- X rays-X rays and CT scans employ radioactive elements which penetrate through the human skin and give a luminiscent view of human body from inside.
Radioactive hazards
If not used in a proper manner, exposure to radiation can cause irrepairable damages to human body as well as other life on Earth.
Below is a list of few of the hazards that are caused by exposure to radioactivity-
- Skin burn– Long exposure to sun can cause burns on skin. This can be observed by tanning that is darkening of the skin. If the skin is exposed to sunlight for a very long time, then it can have permanent damage and sometimes cause skin cancer.
- Radiation burns- When a person comes in direct contact with radioactive material, depending on the amount of exposure to this radiation, he/she can get radiation burns. The skin gets burnt due to the high penetration power of radioactive elements.
- Acute radiation syndrome- This is an illness caused by intake of high amount of radiation in a very short amount of time.
- Cancer– Radiation can cause cancer in our bodies.
- Cardio Vascular diseases– Excessive radiation causes cardio vascular diseases which can be there for entire lifetime and can be passed on genetically.
- Radiation cloud– Atomic blasts leave a huge radiation cloud in the atmosphere thus polluting the atmosphere with radioactive elements. These radioactive clouds then come down in the form of rain.
- Loss of life on Earth– Due to radiation, the innocent plants and animals die because they are unaware of the threats caused by radiation in their bodies.
- Lengthy half life of radioactive materials– Once there is a radioactive leak in an area, it has to be completely sealed for thousands of years as the half life of radioactive elements is far more than human lives. Thus to curb the effect of radiation, the entire population has to shifted and the area needs to be sealed.
Recent Posts
Have Verb In Past Tense: 7 Facts You Should Know
Have in English language is one of the primary auxiliary verbs. We shall explore in this article, how the base form of the verb 'have' is changed to be used in past tense. The past tense form...
Source: https://lambdageeks.com/radioactive-decay-types/
0 Response to "What is Radioactive Decay What is Radioactive Decay Easy Steps"
Post a Comment